Water Treatment
Solutions
Expert Contaminant Removal In The US and The Caribbean
When water quality threatens operations, compliance, or safety, Carver Water Technology delivers proven solutions. From municipal utilities to industrial facilities, we've built our reputation on eliminating contaminants that disrupt business, damage equipment, and violate drinking water standards.
Based in Tampa with coverage across the Southeastern United States and the Caribbean, our team of water treatment professionals responds to your water challenges with the same urgency you face—because we know that in your business, water problems don't wait.
Water Treatment
Solutions
Expert Contaminant Removal In The US and The Caribbean
When water quality threatens operations, compliance, or safety, Carver Water Technology delivers proven solutions. From municipal utilities to industrial facilities, we've built our reputation on eliminating contaminants that disrupt business, damage equipment, and violate drinking water standards.
Based in Tampa with coverage across the Southeastern United States and the Caribbean, our team of water treatment professionals responds to your water challenges with the same urgency you face—because we know that in your business, water problems don't wait.
Understanding Water Contaminants: What's Really in Your Water?
Water quality issues aren't always visible. That cloudy appearance or metallic taste signals deeper problems—contaminants that corrode pipes, foul membranes, violate regulations, and cost you money every single day they remain untreated.
The water flowing through your facility right now likely contains multiple contaminant categories. Some you can see. Most you can't. All of them have solutions
Physical Contaminants: The Visible Enemies
What They Are
Physical contaminants are particles suspended in water:
sediment
sand
silt
rust
organic matter
even plastics and microplastics
In Florida and the Southeast, physical contaminants enter water systems through aging infrastructure, storm runoff, construction activity, and high turbidity source water from rivers and surface supplies.
Turbidity measures suspended particle concentration, reported in Nephelometric Turbidity Units (NTU). Total Suspended Solids (TSS) quantifies particle mass in milligrams per liter (mg/L). Your source water turbidity determines which treatment approach delivers reliable, cost-effective results.
Why they matter: Suspended solids damage pumps, clog pipes, foul membranes, and make water aesthetically unacceptable.
For industrial operations, turbidity interferes with processes requiring clean water.
For municipalities, high turbidity violates Safe Drinking Water Act standards and erodes public trust.




Physical Treatment Solutions
Multimedia Filtration
Layered beds of anthracite coal, sand, and garnet remove particles through depth filtration. Water flows downward through progressively finer media layers, trapping particles throughout the bed depth rather than just at the surface.
Multimedia filters handle turbidity ranging from 5-50 NTU, producing filtered water with turbidity below 1 NTU. Design flow rates typically range from 3-8 gallons per minute per square foot of filter area (gpm/ft²), balancing filtration efficiency with run time between backwash cycles.
When backwash cycles complete, the filter returns to service within minutes. We design multimedia filters with automated backwash controls triggered by differential pressure, run time, or turbidity breakthrough—ensuring consistent performance without operator intervention.
Applications: Municipal water treatment, industrial process water pretreatment, groundwater treatment for iron and manganese removal, membrane system pretreatment.
Greensand Filters
Manganese greensand combines filtration with oxidation, removing dissolved iron and manganese that cause staining, taste issues, and turbidity. The greensand media catalyzes oxidation reactions, converting dissolved metals into particles that trap in the filter bed.
Greensand filters handle iron up to 10-15 mg/L and manganese up to 5 mg/L—common concentrations in Florida groundwater. Periodic regeneration with potassium permanganate restores the media's oxidizing capacity.
Applications: Well water treatment for homes and small commercial systems, iron and manganese removal for municipalities using groundwater sources.
Cartridge and Bag Filtration
Disposable or cleanable cartridge filters provide fine filtration from 0.5 to 50 microns, removing particles that pass through multimedia filters or serving as standalone treatment for low-turbidity water.
Cartridge filters protect sensitive equipment—RO membranes, pumps, control valves, and spray nozzles—from particle damage. We select cartridge micron ratings based on your water quality and equipment protection requirements, balancing filtration efficiency with reasonable cartridge life.
Bag filters handle higher flow rates and larger particles (5-200 microns) in industrial applications with variable water quality. Quick change-out and lower cost per gallon treated make bag filters effective for applications with high particle loading.
Applications: RO system pretreatment, point-of-use equipment protection, industrial process water polishing, final filtration before critical equipment.
Clarification and Settling
For source water with very high turbidity (>50 NTU), chemical coagulation followed by clarification removes the bulk of suspended solids before filtration. Coagulants like aluminum sulfate (alum) or ferric chloride destabilize particles, forming larger flocs that settle in clarifiers.
Clarification reduces turbidity from hundreds or thousands of NTU down to 10-20 NTU. Downstream multimedia filtration then achieves final treated water turbidity below 1 NTU.
Applications: Surface water treatment for municipalities, industrial facilities using river or lake water, storm water treatment.
Carver Water Technology designs, installs, and maintains filtration systems from small commercial cartridge filters to large municipal multimedia filter installations. Our same-day emergency service across Central Florida ensures your filtration systems perform when you need them.
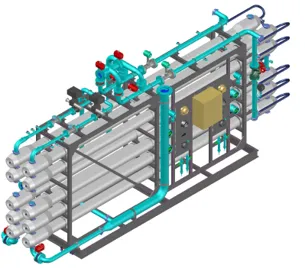



Membrane Filtration Systems: Precision Contaminant Removal
Membrane technology represents the most versatile and effective treatment approach for removing dissolved and suspended contaminants. Membrane filtration uses semi-permeable barriers to separate contaminants based on molecular size and charge. Different membrane types remove different contaminant categories, from suspended particles down to dissolved ions.
Membrane systems operate under pressure, forcing water through microscopic pores while rejecting contaminants that discharge to drain as concentrate or backwash. Proper pretreatment, operation, and maintenance determine whether membrane systems deliver years of reliable service or fail prematurely.
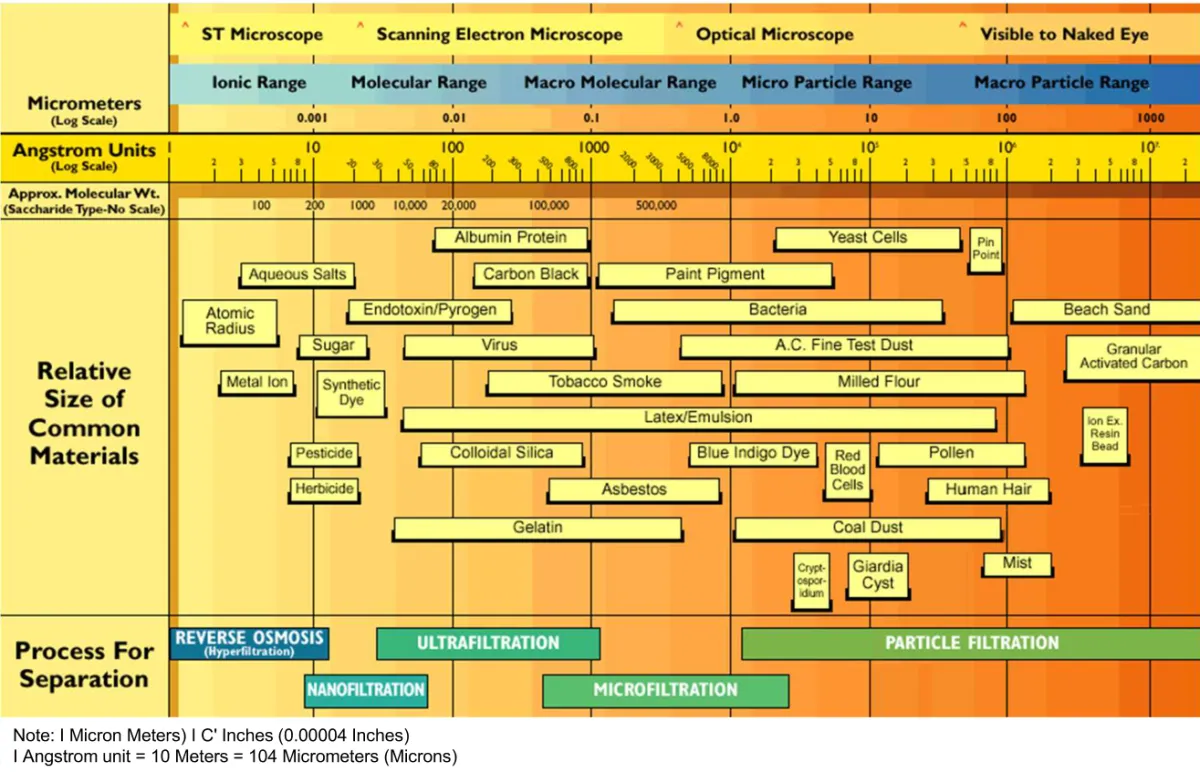
Microfiltration (MF)
Pore Size: 0.1 to 10 microns
What It Removes: Suspended solids, algae, bacteria, and some protozoa. MF membranes provide absolute barriers against particles larger than the membrane pore size.
Operating Pressure: 5-30 PSI (low pressure compared to other membrane types)
Applications:
Pretreatment for reverse osmosis and nanofiltration systems
Turbidity reduction from surface water sources
Bacteria removal for groundwater under direct influence of surface water
Industrial process water clarification
MF systems typically operate in cross-flow configuration, where feed water flows parallel to the membrane surface. This creates shear forces that sweep particles away from the membrane, reducing fouling and extending operating cycles between cleanings.
Advantages: Low energy consumption, high flow rates per membrane area, effective solids removal without chemicals.
Limitations: Does not remove dissolved contaminants. Requires pretreatment to prevent fouling from oils, organics, and scaling compounds.
Ultrafiltration (UF)
Pore Size: 0.01 to 0.1 microns (ten times finer than MF)
What It Removes: Everything MF removes plus viruses, colloidal particles, proteins, and some large organic molecules. UF provides absolute barriers against microbiological contaminants.
Operating Pressure: 10-100 PSI
Applications:
Pretreatment for reverse osmosis systems (produces high-quality RO feedwater with low fouling potential)
Municipal drinking water treatment from surface water sources
Virus removal without chemical disinfection
Food and beverage processing (protein concentration, product clarification)
Pharmaceutical manufacturing (sterile water production)
UF membranes remove turbidity to non-detect levels while eliminating pathogens. This makes UF an effective alternative to conventional coagulation-sedimentation-filtration for surface water treatment, particularly for small and medium-sized utilities.
Advantages: Absolute pathogen barrier, low chemical usage, small footprint compared to conventional treatment, consistent treated water quality regardless of source water variability.
Limitations: Does not remove dissolved salts, metals, or small organic molecules. Capital cost higher than conventional filtration.
Carver Water Technology designs UF systems for municipal and industrial applications requiring reliable pathogen removal and turbidity reduction. Our UF maintenance includes membrane integrity testing, chemical cleaning protocols, and performance optimization.
Nanofiltration (NF)
Pore Size: 0.001 microns (molecular-level separation)
What It Removes: Everything UF removes plus hardness (calcium and magnesium), sulfates, natural organic matter, some nitrates, and many pesticides. NF removes multivalent ions (2+ charge) while allowing monovalent ions (1+ charge) to pass through partially.
Operating Pressure: 50-150 PSI
Key Characteristic: NF softens water without complete demineralization. This produces water with reduced hardness and improved taste while retaining beneficial minerals and maintaining reasonable conductivity.
Applications:
Water softening for municipalities seeking alternatives to ion exchange softening (no salt regeneration required)
Color and organic removal from surface water
Sulfate reduction in groundwater
Industrial process water where partial demineralization is desired
Pretreatment before reverse osmosis to reduce scaling potential
Advantages: Removes hardness without salt usage, lower operating pressure than RO (reduced energy costs), selective removal of specific contaminants.
Limitations: Does not provide ultrapure water. Not effective for complete salt removal or rejection of small organics and monovalent ions.
Reverse Osmosis (RO)
Pore Size: 0.0001 microns (removes molecules)
What It Removes: 95-99% of dissolved salts, heavy metals, nitrates, fluoride, arsenic, PFAS, pharmaceuticals, and most organic compounds. RO produces high-purity water with total dissolved solids (TDS) typically below 10 mg/L from source water exceeding 1,000 mg/L.
Operating Pressure: 150-400 PSI (brackish water RO), 800-1,200 PSI (seawater RO)
How It Works: Applied pressure overcomes osmotic pressure, forcing water molecules through the membrane while rejecting dissolved contaminants that concentrate and discharge as brine.
Applications:
Ultrapure water for dialysis, laboratories, and pharmaceutical manufacturing
Boiler feedwater for power generation and industrial steam systems
Food and beverage production (ingredient water, ice machines, coffee/tea brewing)
Spot-free rinse water for car washes and vehicle care
Drinking water treatment for high-TDS groundwater
Industrial process water requiring low conductivity
Desalination of brackish groundwater and seawater
Critical Design Factors:
Pretreatment: RO membranes require proper pretreatment to prevent fouling and scaling. We design pretreatment including:
Multimedia filtration or cartridge filtration (remove suspended solids)
Water softening or antiscalant dosing (prevent scaling from calcium, magnesium, barium, strontium)
Activated carbon filtration (remove chlorine that damages polyamide membranes)
Acidification (adjust pH to prevent carbonate scaling)
Recovery Rate: Percentage of feed water that becomes product water. Higher recovery reduces water waste but increases scaling risk in the concentrate stream. We optimize recovery based on your source water chemistry and regulatory requirements for concentrate disposal.
Membrane Selection: Different membrane types provide varying rejection rates for specific contaminants. We select membranes based on your target contaminants and water quality objectives.
Cleaning and Maintenance: RO membranes require periodic cleaning to remove fouling and restore flux. Our maintenance programs include:
Performance monitoring (permeate flow, quality, and pressure drop)
Chemical cleaning when normalized performance declines 10-15%
Membrane element replacement when cleaning no longer restores performance
System optimization to prevent future fouling
Carver Water Technology designs, installs, and maintains RO systems ranging from small commercial units (100 GPD) to large industrial and municipal systems (100,000+ GPD). Our RO expertise includes troubleshooting chronic fouling issues, optimizing recovery rates, and extending membrane life through proper operation.
Physical Separation Guide: Particle Sizes
Carver Water Technology designs, installs, and maintains Brackish and Seawater RO systems ranging from small commercial units (2,500 GPD+) to large industrial and municipal systems (100,000+ GPD).
Our pre-designed Brackish Water RO units (BWRO) from 2,500 GPD to 15,000 GPD are for sale online now!
Our membrane expertise includes performance troubleshooting, cleaning protocols, and replacement strategies that maximize your investment.
Our same-day service in Tampa and across Florida ensures your Reverse Osmosis systems perform when you need them.
Chemical & Dissolved Metal Contamination: Treating What You Can't See

The Problem
Dissolved contaminants pass through conventional filtration unaffected. You can't see them, but they corrode pipes, damage equipment, violate health standards, and create long-term liability.
Heavy Metals
Lead, mercury, arsenic, chromium, cadmium, and copper enter water through industrial discharge, mining activities, corroding pipes, and naturally occurring geological formations. In the Southeast, arsenic in groundwater aquifers and lead from aging municipal infrastructure create ongoing compliance challenges.
Health Impact: Heavy metals accumulate in body tissues, causing neurological damage, kidney failure, developmental delays in children, and cancer. EPA Maximum Contaminant Levels (MCLs) are measured in parts per billion—your treatment system must achieve near-complete removal to protect public health.
Hardness Minerals
Calcium and magnesium make water "hard." While not health threats, hardness minerals destroy equipment and waste money every single day they remain untreated.
The Real Cost: Scale deposits reduce heat exchanger efficiency by 25-40%, driving energy costs up while equipment output drops. Boilers, cooling towers, water heaters, dishwashers, and ice machines suffer shortened lifespans. In Florida, where water hardness commonly exceeds 300 mg/L as CaCO₃, untreated hard water costs thousands in premature equipment replacement and maintenance.
Scale clogs pipes, fouling RO membranes and reducing system capacity. Soap and detergent effectiveness drops in hard water, increasing chemical costs in laundries and industrial cleaning operations.
Nitrates and Phosphates
Agricultural runoff, septic systems, and fertilizer use load Florida groundwater with nutrients. Nitrates above 10 mg/L violate drinking water standards and cause methemoglobinemia (blue baby syndrome) in infants. For operations discharging to surface water, nutrient limits protect sensitive watersheds like the Everglades and Florida Springs.
PFAS and Emerging Contaminants
Per- and polyfluoroalkyl substances (PFAS), volatile organic compounds (VOCs), pharmaceuticals, and microplastics represent evolving regulatory challenges. EPA continues tightening limits as health research reveals risks at lower concentrations. Treatment systems installed today must handle contaminants that weren't regulated yesterday.
The Solutions: Removing Dissolved Contaminants
Chemical Dosing and Precipitation: Targeted Treatment
Chemical treatment converts dissolved contaminants into forms that settle, filter out, or remain stable in distribution systems. Precise chemical dosing requires understanding water chemistry, regulatory limits, and how your water will react.
pH Adjustment:
Water pH affects contaminant solubility, corrosion rates, disinfection efficiency, and scaling potential.
Acid Dosing (Sulfuric, Hydrochloric): Lowers pH to prevent calcium carbonate scaling in RO systems and membranes. Also adjusts pH for optimal coagulation and corrosion control.
Caustic Dosing (Sodium Hydroxide): Raises pH for corrosion control in distribution systems, preventing lead and copper leaching from pipes and fixtures. Also used for heavy metal precipitation and post-RO pH adjustment.
CO₂ Injection: Lowers pH without increasing total dissolved solids—important for RO pretreatment where sulfuric acid would add sulfate that increases RO concentrate disposal costs.
Heavy Metal Precipitation:
Lime (calcium hydroxide) or caustic raises pH above 9-10, precipitating most heavy metals as hydroxides that settle in clarifiers or filter out. Effective for treating industrial wastewater and contaminated groundwater before discharge or reuse.
Sulfide precipitation (using sodium sulfide) removes heavy metals to lower concentrations than hydroxide precipitation, meeting stringent discharge limits for facilities treating metals-contaminated water.
Coagulation and Flocculation:
Aluminum sulfate (alum), ferric chloride, and polymers destabilize colloidal particles and dissolved organic matter. After coagulation, particles aggregate into larger flocs that settle or filter out.
Critical for treating high-turbidity surface water and removing natural organic matter that causes disinfection byproduct formation and aesthetic issues.
Corrosion and Scale Inhibitors:
Polyphosphates and proprietary blends prevent scale formation without removing hardness. These chemicals sequester minerals, preventing precipitation in pipes and equipment.
Used in cooling towers, RO system pretreatment, and distribution systems where softening isn't practical. Significantly less expensive than softening but doesn't provide the same equipment protection benefits.
Chemical Feed System Design:
Carver Water Technology designs chemical feed systems with:
Accurate metering pumps sized for your flow rates and dosing requirements
Proper chemical storage with secondary containment meeting regulatory requirements
Flow-paced dosing that adjusts chemical feed rates based on actual water flow
Safety interlocks that shut down chemical feed if flow stops
Monitoring and alarms for tank levels, pump operation, and system faults
We maintain chemical feed systems for municipal and industrial clients, ensuring consistent dosing, regulatory compliance, and safe operation.

We maintain chemical feed systems for municipal and industrial clients, ensuring consistent dosing, regulatory compliance, and safe operation.


Water Softeners: Protecting Equipment and Processes
Water Softeners: Hardness Removal
Ion exchange "softeners" remove calcium and magnesium, replacing them with sodium ions. Softening prevents scale formation throughout your facility without requiring point-of-use treatment at every piece of equipment.
How Softeners Work:
Feed water flows through resin beds loaded with sodium ions. Calcium and magnesium ions exchange with sodium ions on the resin. When resin capacity exhausts (typically after treating 3,000-8,000 gallons per cubic foot of resin depending on hardness), brine solution (10-12% salt) regenerates the resin. Calcium and magnesium flush to drain, resin reloads with sodium, and the softener returns to service.
Modern commercial softeners use demand-initiated regeneration, monitoring actual water usage and regenerating based on treated volume rather than fixed time intervals. This minimizes salt usage and eliminates water waste from unnecessary regenerations.
Softener Sizing:
We size softeners based on peak flow requirements and daily water consumption. Duplex (two-tank) systems ensure continuous softened water availability—one tank treats water while the other regenerates. For facilities with variable demands, we design systems with staging controls that operate additional tanks only when needed, reducing energy and maintenance costs.
Commercial Softener Applications:
Boiler feedwater: Scale-free boilers maintain heat transfer efficiency and extend tube life
Cooling towers: Softened makeup water reduces blowdown requirements and scaling in heat exchangers
Car washes: Protects RO/DI systems producing spot-free rinse water
Commercial laundries: Improves detergent efficiency and extends linen life
Food service: Prevents scale in dishwashers, ice machines, steamers, and coffee equipment
HVAC systems: Protects chillers, boilers, and heat exchangers in commercial buildings
Brine Tank Management:
Salt delivery, storage, and brine tank maintenance are critical for reliable softener operation. We provide salt delivery service, ensuring your system never runs out. Our maintenance programs include brine tank cleaning to prevent salt bridging and mushing that disrupts regeneration.
Carver Water Technology installs and services commercial softeners throughout Tampa, Orlando, and Central Florida. Our clients include Hillsborough County Fire Departments, where reliable softeners protect critical equipment that firefighters depend on daily. We maintain softener systems for operations ranging from small commercial buildings to large industrial facilities processing hundreds of thousands of gallons daily.
Ion Exchange Systems: Selective Contaminant Removal
Beyond softening, ion exchange resins remove specific dissolved contaminants through selective exchange reactions. Different resin types target different contaminants:
Nitrate-Selective Resins: Remove nitrate while minimizing sulfate and chloride removal. After exhaustion, brine regeneration restores capacity. We design nitrate IX systems for small water systems and individual well owners where RO is cost-prohibitive.
Heavy Metal Resins: Selective resins remove specific metals (arsenic, lead, copper) from drinking water. Some applications use single-use resins that require disposal rather than regeneration when treating high concentrations.
Applications: Point-of-entry treatment for homes and small facilities, small municipal systems treating specific contaminants, industrial applications requiring selective removal without demineralization.

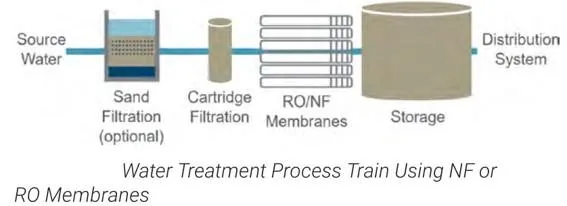
Reverse Osmosis & Nanofiltration: Dissolved Contaminant Removal
For removing heavy metals, nitrates, PFAS, and achieving ultrapure water, membrane systems provide the most effective treatment. RO and NF were covered in detail in the Membrane Systems section above—they excel at removing dissolved contaminants that softeners and conventional treatment cannot address.
Key Applications for Dissolved Contaminants:
Heavy metal removal (arsenic, lead, chromium): RO achieves 95-99% rejection
Nitrate removal: RO removes nitrates to non-detect levels
PFAS removal: RO with high-rejection membranes removes PFAS below detection limits
Total dissolved solids reduction: RO produces water with <10 mg/L TDS from source water exceeding 1,000 mg/L
Hardness removal without salt: NF softens water while retaining some beneficial minerals
Carver Water Technology installs and services commercial softeners throughout Tampa, Orlando, and Central Florida. Our clients include Hillsborough County Fire Departments, where reliable softeners protect critical equipment that firefighters depend on daily.
We maintain softener systems for operations ranging from small commercial buildings to large industrial facilities processing hundreds of thousands of gallons daily.
Biological Contamination: Eliminating Health Threats
The Problem
Bacteria, viruses, and protozoa threaten public health and violate Safe Drinking Water Act standards. In the Southeast's warm, humid climate, biological growth thrives in untreated water systems.
Common Waterborne Pathogens:
Bacteria: E. coli, Legionella, Pseudomonas, and coliform bacteria indicate fecal contamination or allow pathogen growth in distribution systems.
Viruses: Norovirus, hepatitis A, rotavirus, and enteroviruses cause waterborne disease outbreaks.
Protozoa: Giardia and Cryptosporidium resist chlorine disinfection and cause severe gastrointestinal illness.
Health and Regulatory Impact:
Biological contamination causes waterborne disease outbreaks that sicken communities and create liability. For facilities serving vulnerable populations—hospitals, dialysis centers, schools, nursing homes—biological contamination isn't just a violation; it's a crisis.
EPA's Surface Water Treatment Rule requires 99.9% (3-log) removal/inactivation of Giardia and 99.99% (4-log) removal/inactivation of viruses. Groundwater systems under direct influence of surface water face similar requirements.
Even when source water meets microbiological standards, distribution system issues—biofilm formation, cross-connections, low disinfectant residual—create contamination problems that generate boil water notices and erode public confidence.

The Solutions: Biological Contaminant Control

Granular Activated Carbon (GAC): Organic Matter Removal
While GAC doesn't directly kill pathogens, it plays a critical role in biological contamination control by removing organic compounds that serve as nutrients for bacterial growth and interfere with disinfection.
Natural organic matter (NOM) in source water reacts with chlorine to form disinfection byproducts (trihalomethanes and haloacetic acids) that violate drinking water standards. GAC removes NOM before disinfection, reducing disinfection byproduct formation while lowering chlorine demand.
Biofilm control: By removing organic compounds that bacteria feed on, GAC reduces biofilm formation in distribution systems. Less biofilm means lower coliform counts and more stable residual disinfectant concentrations.
Taste and odor: GAC removes organic compounds causing earthy, musty, or chlorinous tastes that drive customer complaints—even when water is microbiologically safe.
How GAC Works:
Activated carbon's massive surface area (over 1,000 square meters per gram) adsorbs organic molecules. Water flows through the carbon bed, and organic compounds bind to the carbon surface through physical and chemical attraction.
Contact Time: Empty bed contact time (EBCT) determines removal efficiency. For drinking water treatment, EBCT typically ranges from 7.5 to 15 minutes. Longer contact time improves removal of difficult-to-adsorb compounds but increases capital cost.
Carbon Replacement: Eventually carbon saturates with organics and requires replacement or regeneration. We monitor treated water quality—UV absorbance at 254 nm (UV254) or total organic carbon (TOC)—to schedule carbon replacement before breakthrough occurs.
GAC Applications in Biological Control:
Pre-disinfection treatment to remove NOM and reduce chlorine demand
Post-disinfection treatment to remove chlorine taste while maintaining biological stability through nutrient removal
Point-of-use treatment in healthcare facilities requiring low-endotoxin water
Carver Water Technology installs and services commercial softeners throughout Tampa, Orlando, and Central Florida. Our clients include Hillsborough County Fire Departments, where reliable softeners protect critical equipment that firefighters depend on daily. We maintain softener systems for operations ranging from small commercial buildings to large industrial facilities processing hundreds of thousands of gallons daily.
Ultraviolet (UV) Disinfection: Chemical-Free Pathogen Inactivation
UV light at 254 nanometers destroys pathogen DNA, inactivating bacteria, viruses, and protozoa without adding chemicals to water. UV provides reliable disinfection that produces no disinfection byproducts and requires no contact time beyond the few seconds water spends in the UV chamber.
Why UV Disinfection:
Effective against chlorine-resistant organisms: Cryptosporidium and Giardia resist chlorine but UV inactivates them at standard drinking water doses (40 mJ/cm²).
No disinfection byproducts: Unlike chlorine, UV doesn't react with organic matter to create regulated trihalomethanes (THMs) or haloacetic acids (HAAs).
No chemical handling: Eliminates safety concerns from storing and handling chlorine gas or sodium hypochlorite.
No impact on water chemistry: UV doesn't affect pH, taste, odor, or mineral content.
Instant treatment: Disinfection occurs in seconds as water flows through the UV chamber. No retention tanks or contact time requirements.
How UV Systems Work:
Low-pressure or medium-pressure mercury lamps generate UV light at 254 nm wavelength. Water flows through a chamber surrounding the lamps. UV light penetrates microbial cells, forming thymine dimers that prevent DNA replication. Organisms cannot reproduce or cause infection.
UV Dose: Measured in millijoules per square centimeter (mJ/cm²). Drinking water typically requires 40 mJ/cm² dose. Wastewater applications may require higher doses.
Critical Design Factors:
UV Transmittance (UVT): Dissolved iron, manganese, organics, and turbidity absorb UV light, reducing dose delivery. Source water must achieve minimum 75% UVT (preferably >85%) for reliable UV disinfection. Pretreatment—filtration, iron removal, or GAC—may be required.
Flow Rate: Manufacturers rate UV systems for maximum flow at target dose. Exceeding rated flow reduces residence time and delivered dose, compromising disinfection.
Lamp Aging: UV output degrades over lamp life (typically 9,000-12,000 hours). Systems must account for end-of-lamp-life output when calculating dose delivery.
Fouling: Mineral scale, biofilm, and iron deposits on quartz sleeves block UV transmission. Systems require periodic cleaning—manual or automated mechanical wipers maintain sleeve cleanliness.
Monitoring: UV intensity sensors continuously monitor lamp output. Alarms alert operators to low intensity, lamp failure, or fouling issues requiring maintenance.
UV System Maintenance:
We maintain UV systems with:
Annual lamp replacement based on operating hours
Quartz sleeve cleaning to remove fouling
UV intensity sensor calibration
UV transmittance testing to verify pretreatment effectiveness
Validation testing to confirm delivered dose
UV Applications:
Municipal drinking water disinfection (particularly for Giardia/Cryptosporidium inactivation)
Point-of-entry treatment for private wells and small water systems
Industrial process water disinfection
Healthcare facility water treatment
Food and beverage production



Carver Water Technology installs and services UV systems from small residential units to large municipal installations. Our UV expertise includes system sizing, pretreatment design, and troubleshooting low-dose issues.
Chlorine and Chloramine Disinfection: Residual Protection
Chlorine remains the most widely used disinfectant in municipal water treatment. Unlike UV, chlorine provides residual disinfection that persists in distribution systems, preventing bacterial regrowth.
How Chlorine Disinfection Works:
Chlorine (as gas, sodium hypochlorite, or calcium hypochlorite) dissolves in water, forming hypochlorous acid (HOCl) and hypochlorite ion (OCl⁻). HOCl penetrates bacterial cell walls, destroying enzymes and DNA.
Disinfection effectiveness depends on:
Chlorine dose (concentration)
Contact time (CT value = concentration × time)
pH (lower pH produces more HOCl, which disinfects more effectively)
Temperature (warmer water requires less contact time)
Interfering substances (organic matter consumes chlorine, reducing disinfection effectiveness)
Free vs Combined Chlorine:
Free chlorine: Hypochlorous acid and hypochlorite ion. Provides effective disinfection and rapid kill times.
Combined chlorine (chloramines): Chlorine combined with ammonia. Provides more stable residual in distribution systems but disinfects more slowly. Many utilities add ammonia after initial chlorine disinfection to form chloramines, reducing disinfection byproduct formation and maintaining residual to system extremities.
Contact Time Requirements:
EPA requires sufficient contact time to achieve 99.9% (3-log) inactivation of Giardia and 99.99% (4-log) inactivation of viruses. Required CT values vary by pH and temperature—we design contact basins or piping that provides adequate contact time under worst-case conditions.
Chlorine Feed Systems:
Gas chlorine: Used by larger municipalities. Requires specialized safety equipment, leak detection, and operator training. Most cost-effective for high-dose applications.
Sodium hypochlorite (liquid bleach): Safer to handle than gas. Available in 5-12.5% solutions. We design hypochlorite feed systems with chemical-resistant pumps, proper storage, and secondary containment.
Calcium hypochlorite (solid): Dry chemical containing 65-70% available chlorine. Requires dissolution and feed systems. Less common in continuous-feed applications.
On-site generation: Electrochlorination systems generate sodium hypochlorite on-site from salt (NaCl) and electricity. Eliminates chlorine transportation and storage concerns for remote facilities.
Chlorine Residual Monitoring:
Maintaining proper chlorine residual throughout distribution systems prevents bacterial regrowth. We install continuous chlorine analyzers at treatment plants and remote monitoring stations, providing real-time residual data and alarming when residuals drop below target levels.
Disinfection Byproducts:
Chlorine reacts with natural organic matter to form trihalomethanes (THMs) and haloacetic acids (HAAs)—regulated disinfection byproducts linked to cancer and reproductive effects. Systems with high organic source water struggle to maintain adequate chlorine residuals while staying below DBP limits.
DBP Control Strategies:
Remove organics before chlorination (GAC, enhanced coagulation) Switch to chloramines for secondary disinfection (slower DBP formation) Optimize chlorine dose to minimum effective level Minimize water age in distribution systems
Chlorination Applications:
Primary disinfection at municipal water treatment plants Secondary disinfection for residual maintenance in distribution systems Well disinfection and shock chlorination Emergency disinfection during system repairs or contamination events Swimming pool and spa water treatment
Safety and Regulatory Compliance:
Chlorine handling requires strict safety protocols. We design chlorination systems meeting OSHA requirements with proper ventilation and leak detection in chlorine rooms, emergency scrubber systems for gas chlorine facilities, personal protective equipment stations, operator training on safe handling procedures, and compliance with Safe Drinking Water Act disinfection requirements.



Carver Water Technology designs and maintains chlorine feed systems with proper safety interlocks, containment, leak detection, and residual monitoring. We ensure your chlorination system meets regulatory requirements while minimizing disinfection byproduct formation and maintaining distribution system water quality.
Frequently Asked Questions
Q1: What exactly are PFAS and why are they called “forever chemicals”?
PFAS (Per- and Polyfluoroalkyl Substances) are a class of over 4,700 synthetic fluorinated compounds. Their key feature is a fluorine-carbon (C–F) bond, one of the strongest in chemistry, which makes PFAS highly resistant to heat, chemical breakdown, and environmental degradation. This durability means PFAS persist indefinitely in water, soil, and living organisms—hence the name “forever chemicals.”
Q2: Which PFAS compounds are regulated under the new EPA rules?
In 2024, the EPA finalized Maximum Contaminant Levels (MCLs) for several PFAS:
PFOA and PFOS: Set at 4 parts per trillion (ppt) each.
GenX (HFPO-DA) and PFBS: Regulated individually at higher ppt thresholds.
PFNA and PFHxS: Addressed in a combined hazard index approach.
These levels are extremely low, reflecting the toxicity of PFAS even in trace concentrations.
Utilities must monitor and treat water to meet these limits.
Q3: How do PFAS typically enter drinking water systems?
PFAS contamination occurs through several pathways:
Industrial discharges from manufacturing plants.
Wastewater treatment plant effluent, which returns PFAS-laden water to rivers.
Firefighting foam (AFFF) runoff from military bases and airports.
Landfill leachate and biosolids applied to agricultural fields.
Atmospheric deposition from PFAS released through factory emissions.
Once PFAS reach surface water or groundwater, they spread widely and infiltrate municipal supplies.
Q4: Which PFAS treatment technologies are most effective?
There is no single “perfect” technology, but leading options include:
Granular Activated Carbon (GAC): Effective for long-chain PFAS like PFOA and PFOS, but less efficient for short-chain compounds such as GenX.
Ion Exchange (IX): Strong-base anion resins can remove both long- and short-chain PFAS, but resin fouling and PFAS-concentrated brine disposal remain challenges.
Reverse Osmosis (RO) and Nanofiltration (NF): RO provides comprehensive removal of nearly all PFAS, while NF is more energy-efficient but less effective on short-chain PFAS. Both generate brine waste streams requiring careful disposal.
Advanced Oxidation Processes (AOP) and Electrochemical Oxidation: These methods can potentially degrade PFAS at the molecular level, but they are energy-intensive and still emerging technologies.
Carver Water often engineers integrated systems (e.g., GAC + RO + IX polishing) to balance cost, efficiency, and waste management.
Q5: What happens to PFAS after treatment—are they destroyed?
This depends on the method:
Adsorptive methods (GAC, IX): PFAS are captured in the media, which then becomes hazardous waste requiring regeneration or high-temperature incineration.
Membrane methods (RO, NF): PFAS are concentrated into a brine waste stream, typically 15–25% of the treated water volume. Brine disposal (deep well injection, evaporation ponds) is costly and tightly regulated.
Destructive methods (AOP, Electrochemical): Aim to break down PFAS into less harmful byproducts. However, incomplete degradation can lead to toxic intermediates, and these technologies are still under optimization.
Q6: How big is the PFAS treatment market?
According to Bluefield Research, U.S. utilities will spend over $3 billion annually by 2030 on PFAS remediation technologies. Drivers include:
-Growing public health awareness.
-New state and federal mandates under the Safe Drinking Water Act.
-Federal grants funding PFAS technology pilots.
-Legal liabilities faced by manufacturers and utilities.
This makes PFAS the next major challenge for U.S. water utilities.
Q7: What are the biggest challenges with PFAS compliance?
Ultra-low regulatory limits (parts per trillion) require highly sensitive monitoring and treatment.
Short-chain PFAS removal remains technically difficult.
Disposal of PFAS waste (spent media, brine) is costly and environmentally sensitive.
High operational costs for advanced technologies like RO and AOP.
Uncertainty around future regulation—EPA is expected to expand its list of regulated PFAS compounds.
Carver Water’s role is to help utilities choose the right mix of technologies, secure funding, and manage ongoing compliance.
👉 Still have questions?
We're Here To Help!
PFAS isn’t going away—but your community doesn’t have to face it alone.
Let's remove PFAS together!
Innovation
Fresh, creative solutions.
Integrity
Honesty and transparency.

Excellence
Top-notch services.

FOLLOW US
TECHNOLOGY & SERVICES
ABOUT US
CUSTOMER CARE
LEGAL
Copyright 2026. Carver Water . All Rights Reserved.
